Products
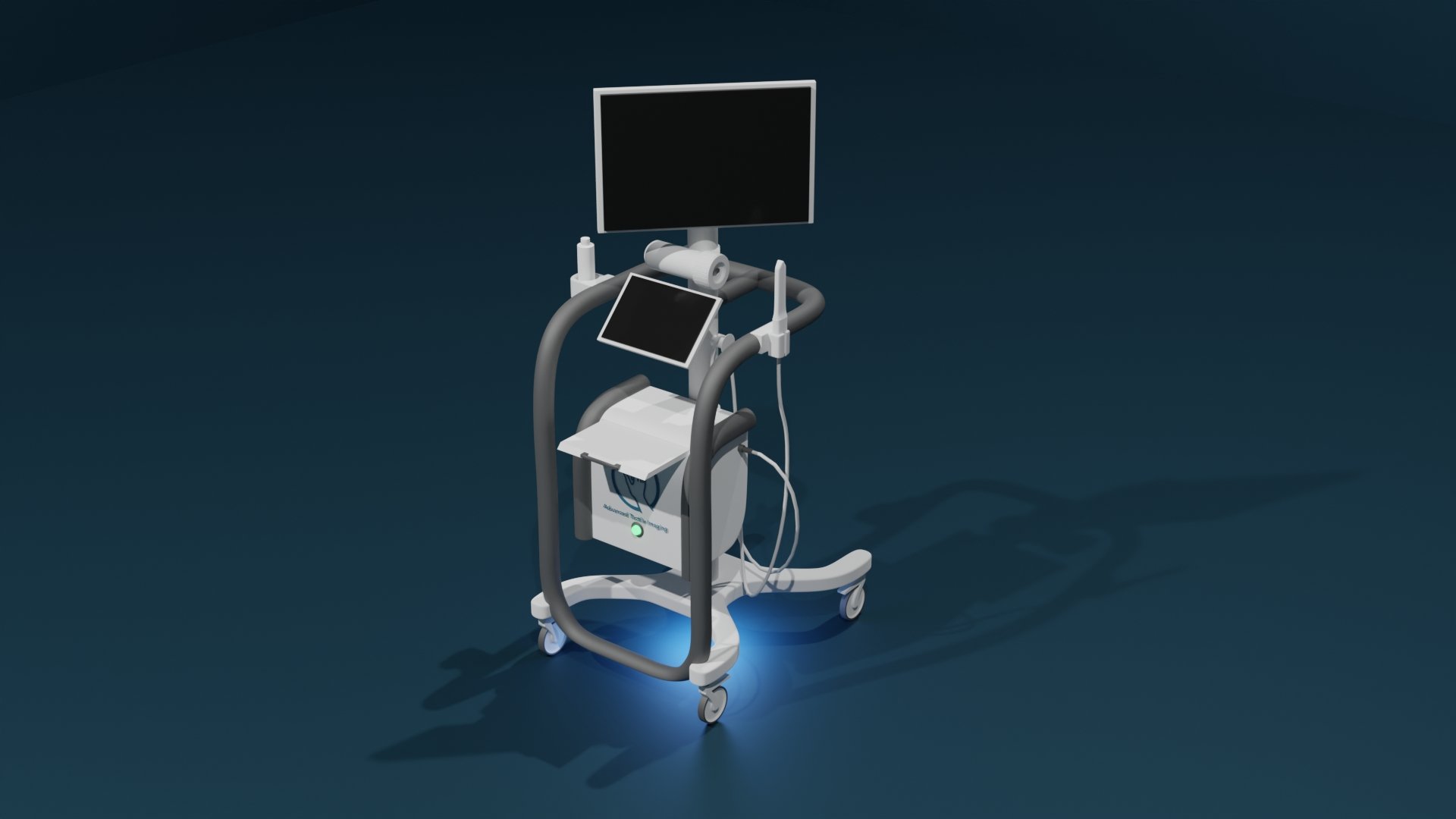
Vaginal Tactile Imager (VTI)
A clinically validated device to aid in diagnosis and evaluation of vaginal and pelvic floor conditions. It provides Biomechanical Integrity score (BI-score) as well as quantitative data for:
Vaginal tissue elasticity
Pelvic floor support
Pelvic muscle contraction
Muscle relaxation
Muscle mobility
CPT Code: 0487T
Biomechanical mapping, transvaginal, with report
FDA Approval (USA)
CE Mark (Europe)
TGA Approval (Australia)
Advancements for
Urogynecology
Gynecology
Physiotherapy
Cosmetic Gynecology
Indications for Use (FDA)
"The Vaginal Tactile Imager obtains a high-resolution mapping of pressures and assesses the strength of pelvic floor muscles within the vagina. It is used in a medical setting to acquire the pressures and store the corresponding data. It also provides visualization, analysis tools and information. The real-time data, as well the analysis information, can then be viewed with an intention of assisting in the diagnosis and evaluation. The device is intended for use by physicians, surgeons and medically trained personnel."
Clinical Utility
The VTI may be used for:
POP (Pelvic Organ Prolapse): To characterize its development in biomechanical terms.
UI (Urinary Incontinence): To quantify pelvic muscle defects that contribute to UI.
Surgery: To provide metrics for pelvic floor surgery outcomes.
Physiotherapy: To monitor pelvic conditions during the course of applied treatment.
RF (Radiofrequency) and Laser treatments: To evaluate vaginal conditions before and after laser or RF treatment.
Military and Sport: To monitor pelvic floor changes under heavy, repetitive loads.
Developed with financial support of the National Institute on Aging (NIA) of the National Institutes of Health, USA.
Application of the Vaginal Tactile Imager (VTI) in Occupational Health Context:
The VTI is a diagnostic medical device that provides high-resolution mapping of intravaginal pressure patterns and quantifies pelvic floor muscle function. Its primary use is in the assessment, early detection, and monitoring of pelvic floor disorders, including stress urinary incontinence, pelvic organ prolapse, and muscle dysfunction—conditions that can significantly impact a woman’s physical health, productivity, and quality of life, particularly in the workforce.
In the context of occupational health and workplace well-being, the VTI can be utilized in the following ways:
Occupational Health Screenings: Employed in preventive health screenings for women in physically demanding roles (e.g., nursing, caregiving, manufacturing), the VTI supports early identification of pelvic floor dysfunction, enabling timely interventions to reduce long-term morbidity and absenteeism.
Corporate Health Programs: The device may be integrated into employee wellness and women’s health programs as part of personalized assessments aimed at promoting musculoskeletal and urogynecological health.
Company Medical Services: On-site or affiliated occupational health clinics can incorporate the VTI to support ergonomic assessments, return-to-work evaluations, and recovery monitoring for employees affected by pelvic floor disorders due to occupational stress or postpartum challenges.
We believe that supporting women’s pelvic health through such diagnostic tools contributes meaningfully to workplace safety, functional capacity, and gender-specific occupational health strategies.
2. Vaginal Tactile Ultrasound Imager (TIUSv)
A unique device to aid in diagnosis and evaluation of vaginal and pelvic floor conditions. It provides tactile (stress) and ultrasound image (anatomical, strain) fusion using the vaginal probe.
Indications for Use:
“The Vaginal Tactile Ultrasound Imager obtains a high-resolution mapping of pressures, ultrasound images and assesses the strength of pelvic floor muscles within the vagina. It is used in a medical setting to acquire the pressures, ultrasound images and store the corresponding data. It also provides visualization, analysis tools and information. The real time data as well as the analysis information can then be viewed with an intention of assisting in the diagnosis and evaluation. The device is intended for use by physicians, surgeons and medically trained personnel.”
Regulatory status: FDA approved
Developed with financial support of Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health, USA.

